Benzylphosphonic acid hasn’t grabbed headlines like some of its relatives, but it’s always found a way into labs and industries where engines of curiosity run strong. Its roots reach back to the expansion of organophosphorus chemistry in the mid-20th century, driven by the search for novel ligands and coupling agents. Chemists explored attachments of phosphorus atoms onto aromatic rings, landing on structures like benzylphosphonic acid, which brings a unique mix of reactivity and stability. This quiet molecule has crossed the threshold from specialty labs to commercial catalogues, keeping pace with discoveries in both organic synthesis and materials science.
This compound, with the structure C7H9O3P, offers a phosphonic acid group attached to a benzyl group. It comes as a white to off-white powder, soluble in water and polar organic solvents. Suppliers package it with purity above 97% for research and industrial needs, and it can show up under various synonyms, such as alpha-phenylmethylphosphonic acid or (phenylmethyl)phosphonic acid. Stock solutions keep well at room temperature if the container stays tightly closed and away from moisture, ensuring stability over months.
Benzylphosphonic acid features a melting point above 140°C. Water solubility sits at the medium range due to hydrogen bonding from the phosphonic acid group and the hydrophobic push from the benzyl ring. I’ve seen it blend quickly in dimethyl sulfoxide for NMR analysis. The compound resists rapid oxidation, survives gentle heating, and doesn’t fume under normal conditions. Its acid group holds a pKa around 2 for the first proton and 7 for the second—a stepwise acidity typical of phosphonic acids—which shapes its ability to chelate metals or bind to oxides, defining much of its value in research and industry.
Labs receive benzylphosphonic acid in dark amber glass bottles, labeled to show batch number, purity, and storage temperature recommendations. Certificate of analysis often includes NMR and IR spectra to demonstrate identity and exclude trace solvents. European REACH-compliant packaging and US DOT shipping labels assure buyers of regulatory diligence. Barcodes and QR codes on bottles bring up safety data and recommended handling procedures, reflecting the need to anchor chemical handling in a digital workflow rather than loose paper records.
The most common route for synthesizing benzylphosphonic acid starts from benzyl chloride and trialkyl phosphite—an Arbuzov reaction—yielding benzylphosphonate ester, which converts to the acid through hydrolysis with strong mineral acids or base. This classic two-step approach scales easily, and yields push above 80% with some patience in controlling reflux temperatures and hydrolysis conditions. Purification involves repeated crystallizations or chromatography, ensuring the removal of unreacted starting materials for sensitive laboratory and industrial applications.
Benzylphosphonic acid stands ready for multiple chemical adventures. The phosphonic acid group binds tightly to transition metals and metal oxides, leading to surface modification on everything from titanium implants to iron nanoparticles. Derivatization by esterification with alcohols, or amide formation with amines, tailors solubility or linker length for bio-conjugation. Researchers often use this molecule in Suzuki or Sonogashira couplings, benefiting from its stability alongside palladium or copper catalysts. I’ve run reactions tweaking the benzyl ring, adding substituents to see how electronic shifts affect binding, with measurable diversions in NMR signatures and ligand behavior.
Catalogues list benzylphosphonic acid as alpha-phenylmethylphosphonic acid, (phenylmethyl)phosphonic acid, or simply benzylphosphonic acid. These names show up in commercial and academic settings. CAS registry numbers help ensure buyers know what they’re ordering, sidestepping misunderstandings common with less specific chemical names. Multiple suppliers, from Sigma-Aldrich to Tokyo Chemical Industry (TCI), carry this molecule, signaling its relevance across boundaries of research focus.
This compound doesn’t qualify as acutely toxic, yet like many phosphorus compounds it needs respect for its irritant potential. Gloves and eye protection keep accidental exposure low. Fume hoods catch splashes or dust released during weighing and solution prep. Any contaminant with skin or eyes leads to flushing with water and, if inhaled, stepping out for fresh air. Disposal calls for collection in halogenated solvent waste or acid-resistant drums. Safety data sheets (SDS) remind users to keep containers closed and ventilated and reassure about its non-flammable nature. Labs committed to good practice store it in corrosion-resistant cabinets, close to chemical spill kits, and they don’t treat it casually, even if acute hazards stay minimal.
Surface modification eats up a lot of benzylphosphonic acid’s global output. It acts as a coupling agent for attaching organic molecules to metals and metal oxides. Materials scientists use it to coat nanoparticles, changing their dispersibility in water or organic solvents. Biomedical engineers build implant surfaces with this molecule to reduce bacterial attachment and nudge tissue growth. Analytical chemists reach for benzylphosphonic acid to chelate metal ions, refining separation protocols or improving detection in sensors. In organic synthesis, it acts as a starting point for more complex phosphorus ligands—a fundamental component for asymmetric catalysis and cross-coupling processes. Each field leverages the unique chelating and binding profile the phosphonic acid offers, making it more than just another commodity acid.
The last decade has seen an uptick in the design of phosphonic acid derivatives for energy storage. Scientists at national labs reported that benzylphosphonic acid anchors organic frameworks to metal oxides, powering the next phase of battery and supercapacitor materials. The biomedical sector explores it for antibacterial coatings, describing real improvements in stent and joint performance. In environmental chemistry, researchers use it to trap or sense heavy metals, addressing industrial spill risks in rivers. Smart polymers gain new traits from the addition of benzylphosphonic acid groups, with water purification and smart coatings emerging well beyond pilot scale. The literature reflects a wave of patent filings, each staking a claim on a new use-case fueled by this modest yet capable molecule.
Historical toxicology paints benzylphosphonic acid in a mild color. Standard in vitro tests show low acute toxicity to mammalian cells. Animal exposure studies find no evidence of teratogenic effects or bioaccumulation at environmental concentrations. The molecule undergoes metabolic oxidation, breaking down in water and soil without forming persistent or dangerous intermediates. Regulations currently set no remarkable thresholds for workplace exposure, though prudent practice still drives scientists to minimize dust and splashes. Long-term ecological studies continue, especially as more end-uses emerge in environmental, food, and medical applications, and some governments now push for more rigorous chronic exposure data.
As energy and environmental challenges take center stage, benzylphosphonic acid finds new relevance. Battery tech developers need robust, stable linkers, and this compound slides into that gap. Water remediation companies and environmental labs push for safe, selective chelators that don’t leave a heavy environmental mark. Biomedical designers look to thin-film coatings and traceless surface modifications, with increased demand for low-toxicity, high-affinity molecules. Each application demands a skillful match of physical and chemical properties—all things this molecule delivers after decades of testing and refinement. As regulations tighten and green chemistry principles move closer to the mainstream, benzylphosphonic acid offers a bridge between legacy chemistry and new, sustainable science. It’s a chemical well-positioned for a second act, built on a strong, well-documented history.
The story of benzylphosphonic acid in modern material science connects with the rapid expansion of electronics and renewable energy over the past decade. In several research labs, chemists apply this compound as a reliable anchoring agent. Thin films, metal oxide surfaces, or nanoparticles often need something strong to keep functional molecules in place. Benzylphosphonic acid offers a strong phosphonic group that tightly bonds with metal oxides, while its benzyl portion helps support organic modifications. This makes it a go-to molecule for self-assembled monolayers—ordered, one-molecule-thick layers used to fine-tune the surface properties of sensors, catalysts, and even solar cells.
Solar cell research often turns to benzylphosphonic acid when improving efficiency. As someone who has talked with researchers in photovoltaics, they often share that the interface between a metal oxide—like titanium dioxide—and its coating layer is a big hurdle. Molecules like benzylphosphonic acid help bridge the gap. By attaching to the oxide surface, they create a chemical “highway” that helps transport electrons more effectively. This sort of chemistry appears abstract, but its practical impact means squeezing extra percentage points out of a solar panel. Given energy prices and the push for cleaner power, every increase matters.
Many industries turn to coatings to keep metal structures safe from rust and decay. Benzylphosphonic acid serves as part of specialized formulations for anti-corrosion layers. Tanks, pipelines, and machinery in harsh environments face attack from moisture, oxygen, and chemicals. A coating that includes phosphonic acids forms a layer at the microscopic level. Instead of relying on thick paint or heavy barriers, this chemistry creates a shield that locks into the surface. In the field, that can cut repair costs and extend the working life of expensive infrastructure—vital, especially where budgets only stretch so far.
Organic synthesis—the workhorse of developing new drugs, plastics, and specialty chemicals—uses benzylphosphonic acid as a building block. Its combination of a phosphonic acid group and a benzyl ring gives chemists more room to create molecules for pharmaceuticals and agriculture. For work on flame retardants or plasticizers, the phosphonic moiety is valuable for imparting fire-resistant qualities. Having access to tailored molecular components speeds up R&D and opens new doors for tackling persistent challenges, like making materials safer or more environmentally friendly.
Benzylphosphonic acid, like many specialized chemicals, asks for a level of respect. Safety data points to it causing irritation if handled carelessly. Standard lab practice—wearing gloves, goggles, and working in a ventilated space—reduces risks. Manufacturers must provide clear information and training on its hazards, so both workers and the environment stay protected. This aligns with wider regulatory expectations around chemical use, aiming to balance innovation with health and sustainability.
The science behind benzylphosphonic acid might seem complex, but it forms a foundation for practical advances in energy, manufacturing, and technology. As research teams and industries keep looking for more efficient and sustainable solutions, understanding how and why these molecules work will help bridge the gap between high-level chemistry and real-world improvements.
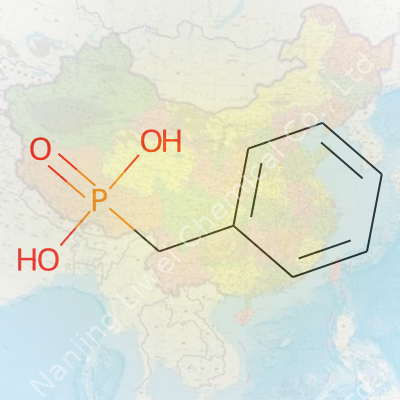
Benzylphosphonic acid, with a molecular formula of C7H9O3P, sits at a crossroads between simple organic chemistry and the more complex world of phosphorus-containing compounds. The structure seems straightforward—a benzyl group, which counts as a phenyl (a six-carbon ring) attached to a –CH2– bridge, linked to a phosphonic acid group. If you’ve handled even basic organic reagents, this combination starts to make sense.
The phosphonic acid part stands for –PO(OH)2. Attach this to a benzyl (C6H5CH2–), and you have C6H5CH2PO(OH)2. This matches up to the formula: seven carbons, nine hydrogens, three oxygens, one phosphorus.
Lay out the compound on paper, and you’ll find the benzyl part bonded through its –CH2– to the phosphorus atom. That phosphorus, in turn, anchors two hydroxyl (–OH) groups and double-bonds to one oxygen. Chemists spot these features right off because they mirror those seen in other phosphonic acids; the difference in this molecule lies in the unique influence of the aromatic group.
Simple line or skeletal diagrams show a six-membered ring, with a single –CH2– arm sticking out. That arm leads to a phosphorus atom double-bonded to oxygen and bound to the two –OH groups. There’s clarity and symmetry in the layout, which is almost comforting for anyone who’s ever struggled through reaction mechanisms.
Whether it belongs on a shelf in a research lab or woven into an industrial process, benzylphosphonic acid wins attention because of its reactivity. That phosphonic acid group isn’t just window dressing—it decides how the molecule interacts in synthesis or with metal ions. I’ve seen how such groups bring a whole new level of versatility, letting researchers build more complex molecules or create stronger links with surfaces for catalysis or material science.
These aren’t just hypotheticals. Studies into compounds like benzylphosphonic acid show they can bind to metal surfaces, making them choice candidates for coatings, corrosion inhibitors, and catalysts. Some research hints at roles in biochemistry, with phosphonic acids mimicking the phosphate groups in DNA or enzymes, though benzyl substitutions aren’t as common in those settings.
Getting benzylphosphonic acid begins with a basic benzyl halide—think of benzyl chloride—reacted with a phosphite ester. A simple example: Treat benzyl chloride with triethyl phosphite, then hydrolyze the resulting ester. The steps take a fair bit of patience, but the outcome is reliable. Seeing this in practice, the transformation goes faster than watching paint dry, but slower than you'd hope during finals week in college. The point is, the chemistry is accessible, which opens up opportunities for labs with even modest resources.
Every compound like benzylphosphonic acid offers a chance to address bigger challenges. Improved surface modification in green technologies, better drug design, and advanced materials all trace roots back to established structures like this one. And while this acid hasn’t hit celebrity status like aspirin or caffeine, it fills an important spot for those exploring the possibilities of phosphorus in organic synthesis.
Reliable data, such as the clear definition of its molecular formula and structure, help newcomers and seasoned researchers alike. Trustworthy research and transparent sourcing set the standard—a key principle for anyone serious about pushing chemistry into new territory.
Chemicals find their way onto storage shelves everywhere, from university labs to small startups dreaming of a breakthrough. Benzylphosphonic acid tends to demand a little more attention than table salt or sugar. It can irritate skin, and the dust gets in the air faster than you’d expect. Unlabeled bottles cluttering the bench do not help anybody, and if humidity sneaks in, so does trouble.
Polyethylene or glass bottles, sealed tight, cut down on moisture and air. Labels ought to be waterproof, and they stand out more under clear tape. More than once, I’ve watched coworkers hunt for clues on faded containers, only to discover a mystery white powder with zero documentation. Risk goes up. It’s a recipe for confusion.
Locking cabinets keep accidents from happening when curious hands reach for something shiny. Larger stock sits on a low shelf in the chemical store, never above shoulder height—no high-stakes juggling act. Sheer experience beats blind confidence. Every mistake seems avoidable in hindsight.
Room temperature works if you avoid temperature swings and direct sunlight. Direct heat wrecks stability and sunlight kicks off slow decay. Store drums or bottles away from vents and windows. Good ventilation clears any stray fumes, and no one likes surprises when unscrewing a lid.
Dedicated acid cabinets keep the fumes from spreading. Combining acids and bases in one spot always causes problems later. Fume hoods serve best for dispensing or making solutions. A splash in the eye punishes shortcuts.
Goggles and gloves sit by the door for a reason. Nitrile gloves protect skin better than latex here. A simple cotton lab coat catches most spills, and long sleeves save skin from pain. Fume hoods get overlooked by new lab staff—sometimes people act like there’s no need until something goes wrong.
If dust scatters, a dust mask or a P100 respirator means you won’t dread your next lungful of air. I still remember a coughing fit after a small spill because nobody wanted to admit the extractor fan wasn’t working. Talk about learning the hard way.
Store waste in the right drum with a clear acid label. Combining acids and unknown organics sounds tempting on a busy afternoon. That shortcut asks for emergencies. Disposal schedules matter, and a logbook avoids confusion. Tossing wrappers or gloves in the regular trash creates long-term problems everyone wants to avoid.
Written protocols help, but real safety comes from paying attention and sharing tips. Regular refreshers make sense, even for seasoned chemists. Nobody wants the job of cleaning up a spill that happened because training fell through the cracks. The phone list for emergencies, printed out and taped near the storage area, keeps problems from growing.
People underestimate how a small mishap with benzylphosphonic acid can derail research or cause harm. Taking fifteen minutes to do things right beats spending weeks waiting for bandages to come off.
Working in a chemistry lab has taught me that skipping safety steps leads to headaches—both literal and figurative. Benzylphosphonic acid isn’t some harmless vinegar. Its properties make it useful for surface treatments and specialty coatings, but they also demand real respect in the workspace. Getting eyes or skin exposed to the stuff turns a regular shift into a trip to the eye wash or worse.
People laugh at goggles until their eyes sting for hours. Long sleeves, tightly woven lab coats, solid nitrile gloves—even shoe covers matter more than they seem, especially when handling powders or corrosive solutions. Gloves without visible tears and safety goggles shouldn’t collect dust; they form the first line of defense. In one crowded student lab, I watched as someone’s torn gloves soaked right through, causing an impressive rash that kept them out of class for days.
I’ve spent hours in labs that smell like a chemical cocktail, and poor air movement only multiplies the hazards. Good fume hoods matter a lot. Vapors may not always be obvious, but inhalation can lead to throat irritation or headaches. If something feels off in the air, turn on the fan or move operations. Not every exposure knocks you flat in minutes—it’s the lingering effects that pile up and burn your sinuses.
Spills happen, especially under pressure. I learned the hard way to avoid grabbing random paper towels as a first instinct. Dedicated spill kits—full of inert absorbents, scraper tools, and neutralizers—belong in reach, not under a pile of boxes. It helps to run through spill drills, so the reaction isn’t to panic but to follow a clear, practiced plan. Scrub suits and basic shoe covers cut down cleanup time, and I remember the relief when a classmate’s quick action stopped a spill spreading past the benchtop.
Shelf space tightens up, but storing benzylphosphonic acid far from incompatible chemicals like strong bases or oxidizers keeps surprises down. Fire-resistant cabinetry feels overkill until the one time something tips and reacts. Always check that containers stay sealed tight; humidity sneaking in or tiny leaks set up future headaches. I keep labels updated, since faded handwriting or mystery bottles add confusion when it counts the most.
Disposing of chemicals used to bother me—until someone poured a harmless-looking compound down a sink and shut down the whole drainage system for days. For benzylphosphonic acid, follow local hazardous waste disposal guidelines closely. Waste labels must show exactly what’s inside. Clean glassware with care, so residues don’t crop up in unexpected runs or cross-contaminate new experiments.
I learned early on that skipping safety refreshers was a shortcut to trouble. Each person handling chemicals gains from hands-on practice. Safety sheets and real-world demonstrations help connect big caution signs to practical steps. People in the lab watch each other’s backs; nobody’s too experienced for a gentle reminder. Questions get answered quickly, and new folks aren’t left guessing in silence.
Chemicals like benzylphosphonic acid demand a thoughtful approach each time they’re handled. By practicing routine precautions and supporting each other, risks shrink dramatically. It’s a sign of pride and professionalism to keep everyone focused on safety as the foundation of real progress in the lab.
Anyone who’s ever tried to clean up after a project in the garage knows how little things add up. Just like a little dirt can ruin a finish, impurities in chemicals like benzylphosphonic acid can throw off big experiments in labs or even hold back processes in industry. Purity isn’t just a number—it's a promise. The stuff researchers trust for sensitive experiments at a university might not come from the same drum as what builders pour into heavy machinery. And there’s a world of difference between the two.
Walk into any chemical storeroom at a research lab, and you’ll find bottles stamped with purity grades—sometimes 98%, sometimes even higher. And for good reason. Scientists count on this accuracy. Trace metals or leftover solvents can ruin a reaction or make results useless. Mistakes can be expensive and months of work can disappear because the acid wasn’t quite up to snuff. I remember talking with a materials chemist who lost weeks because a batch contaminated with iron left unreliable data, forcing a costly restart. No one forgets lessons like that. It’s not just fussy perfectionism. It’s about trust and reproducibility.
Factories and large-scale manufacturers look at purity differently. A hint of something extra in a batch won’t always cause a problem if someone’s treating metal or prepping a surface. Once, touring a phosphate producer, I saw that their standards for products heading to a heavy-duty paint manufacturer didn’t match what you’d see in academic settings. There, they wanted just enough quality so the process worked and costs made sense. Less expensive material often works fine, as long as the “impurities” don’t cause big trouble. Some call this pragmatic—but it really comes down to experience. Over time, manufacturers know just how pure they need their chemicals, and they won’t pay extra for something that doesn't make a difference.
There’s no one answer for everyone. If you’re running a tight research operation or working up a new drug, the catalogs often offer high-purity options, sometimes with certificates that detail heavy metals or moisture content down to the last decimal. These certificates aren’t just paperwork for the archives. Regulatory audits, patent filings, journal submissions—each one demands proof that the ingredients matched the claims.
Outside the lab, technical grade products come at a friendlier price and usually do the job. Industries dealing in surface treatment, water chemistry, or cleaning agents often use them by the ton. That said, cutting corners by using industrial grades in sensitive work rarely pays off. The risks can snowball, leading to equipment fouling, unsuccessful coatings, or unexpected toxicity. From what I’ve seen, buying the highest purity for every job isn’t efficient, but not knowing the minimum requirement leaves you open to expensive problems.
This isn’t an issue solved by picking the “best” grade. Every situation asks for a different tool. Labs can keep their trust in high-purity acids, with documentation to back up every step for publications and audits. Industry can focus on reliable suppliers who know the level that works in big processes. It helps to invest in real sample testing, and open conversations with suppliers always pay dividends. As sourcing quality becomes a bigger concern worldwide, knowing exactly what you need—and getting it—is more important than ever. People who spend the time to get this right rarely regret it. I’ve watched too many teams learn the hard way: the grade of chemical you choose sets the stage for the project that follows.
| Names | |
| Preferred IUPAC name | (phenylmethyl)phosphonic acid |
| Other names |
Benzylphosphonic acid benzylphosphonate Phosphonic acid, benzyl- Benzenemethylphosphonic acid |
| Pronunciation | /ˈbɛn.zɪl.fɒsˈfɒn.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | [4418-26-2] |
| Beilstein Reference | 79018 |
| ChEBI | CHEBI:73985 |
| ChEMBL | CHEMBL429844 |
| ChemSpider | 162162 |
| DrugBank | DB08696 |
| ECHA InfoCard | 03d3a777-e8ae-4103-a019-6791b4ad5498 |
| EC Number | 412-410-0 |
| Gmelin Reference | 66163 |
| KEGG | C06305 |
| MeSH | D017889 |
| PubChem CID | 153080 |
| RTECS number | TB4375000 |
| UNII | 6F1Q2T140N |
| UN number | 3265 |
| CompTox Dashboard (EPA) | DTXSID50895421 |
| Properties | |
| Chemical formula | C7H9O3P |
| Molar mass | 204.15 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.32 g/cm³ |
| Solubility in water | Soluble |
| log P | -1.0 |
| Vapor pressure | Vapor pressure: <0.01 mmHg (25°C) |
| Acidity (pKa) | 2.2 |
| Basicity (pKb) | 13.10 |
| Magnetic susceptibility (χ) | NA |
| Refractive index (nD) | 1.575 |
| Viscosity | Viscosity: 338 cP (25 °C) |
| Dipole moment | 5.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 117.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -546.4 kJ/mol |
| Pharmacology | |
| ATC code | C01EB09 |
| Hazards | |
| Main hazards | Causes severe skin burns and eye damage. |
| GHS labelling | GHS02, GHS07, GHS05 |
| Pictograms | GHS05 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H411 |
| Precautionary statements | P264, P280, P301+P312, P330, P305+P351+P338, P337+P313, P501 |
| NFPA 704 (fire diamond) | 1-2-0-~ |
| Flash point | 222°C |
| Autoignition temperature | 280°C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 940 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1600 mg/kg (rat, oral) |
| NIOSH | SWG3990000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | ACN |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Benzyl phosphonate Benzylphosphonyl dichloride Phenylphosphonic acid Methylphosphonic acid Benzylphosphine oxide |