Phosphorous acid stepped into scientific view during the golden era of 18th-century chemistry. Anton Baumé, a French chemist, first described it after working with phosphorus and water. Since then, chemists have tuned the processes and the understanding of this compound over generations. The early enthusiasm for phosphorus chemistry grew out of its unique reactivity and critical role in food production, leading to deeper exploration of phosphorus-containing acids. Industry didn’t hesitate to bring phosphorous acid into the fold, especially with its clear advantages in making more effective herbicides and fungicides. The journey from laboratory curiosity to an industrial staple tracks closely with increased demand for food security and plant disease management.
Phosphorous acid, chemically known as H3PO3, appears as a clear, colorless crystalline solid at room temperature. Many industries buy it as a liquid solution for convenience during application. This compound doesn’t get confused with phosphoric acid, despite their similar names and roots in phosphorus chemistry. Phosphorous acid stands out for its role as a strong reducing agent and its utility in synthesis and agriculture. Chemists, always after purity and stability, favor this chemical for reactions that need precise control. The agricultural sector, on the other hand, grabs for it as a base ingredient in a wide range of fungicides and systemic plant health products. Some specialty markets use it to make chemicals that change the way metals behave in water systems, especially for corrosion control and water treatment.
Phosphorous acid presents a melting point close to 70 °C and starts breaking down above 180 °C. This chemical dissolves easily in water with a heat release you’ll notice. Its molecular structure, with one phosphorus atom bonded to three oxygens and one hydrogen, determines reactivity and application profile. Unlike many acids, it doesn’t show the same strength when tossing it in water, and you can expect a pH dropping into the acidic range. Its solid form absorbs water easily, so open containers do not last long before caking or dissolving. Most notable is its tendency to give up electrons easily, making it a favorite in reduction reactions and as a starting point for making phosphonates.
Any industrial supplier labels phosphorous acid with precise weight percent, often above 75% solution in water. Purity usually stays above 99% in solid deliveries. Standard labeling never skips over hazard warnings, especially about chemical burns and respiratory irritation. Storage instructions focus on keeping it away from bases and oxidizers. Technical data sheets avoid ambiguity, laying out density at different concentrations, expected shelf life under normal warehouse conditions, and safe disposal methods. The global supply chains certify each batch, based on guidance from regulators in the United States, European Union, and other major economies.
Factories make phosphorous acid mainly by reacting phosphorus trichloride (PCl3) with water. The process calls for careful handling—PCl3 fumes cause nasty burns, and the reaction creates hydrochloric acid as a byproduct, which itself needs special handling. An older method involves direct oxidation of elemental white phosphorus in water, but today most production stays with the trichloride route for better control and safety. Plant engineers monitor temperature, acid concentration, and byproduct removal to keep final product grade high. In my work with chemists, keeping line workers safe through training matters as much as tweaking the process.
Phosphorous acid does not just sit on the shelf; it serves as an active ingredient in a surprising variety of chemical reactions. In reduction chemistry, it turns metal ions or organics into their simpler forms. Some labs use it as a starting material for phosphonate synthesis, connecting phosphorus to carbon for new molecules that block or enhance biological enzymes. This family of products powers several herbicides and water conditioning agents. In special modifications, chemists swap out hydrogen atoms for organic chains, tuning everything from water solubility to activity inside living plants. These reactions demand calm hands and careful work due to the heat and byproducts that can escape during the process.
Chemists may call phosphorous acid by several names. A common synonym is orthophosphorous acid. Product catalogs list it as H3PO3, Phosphonic Acid (though that term causes confusion with a related family), and Phosphorous acid solution. In trade, names and even abbreviations matter for avoiding costly mix-ups, and seasoned buyers look for assured purity and clear labeling to avoid errors. Sometimes labels include language from local regulations or international transport codes, especially for cross-border shipments. Safety data sheets always match these names to CAS number 10294-56-1, so that buyers and users know exactly what’s in the barrel or drum.
Phosphorous acid doesn’t mess around with safety—it’s a potent acid and a skin irritant with an ability to cause burns on direct contact. Respiratory hazard increases during handling and mixing. Plant safety teams set up strict containment and demand personal protective equipment: chemical-resistant gloves and face shields, and sometimes breathing masks, become standard. Warehouses store it away from oxidizers and alkali to avoid dangerous reactions. Workers get specific training before handling bulk shipments or process chemistry. Emergency showers, eye-wash stations, and ventilation systems ensure safe operations. Having worked on chemical safety plans, I’ve learned that emphasizing clear labeling, quick clean-up drills, and spill containment makes a big difference in accident reduction. Regulatory authorities demand detailed transport documentation and limit allowable weights per container to avoid accidental releases.
Farmers and agricultural companies make the biggest use of phosphorous acid. Its power as a systemic fungicide stands out—especially against downy mildew, root rot, and other plant diseases that can destroy entire crops. Grapevine growers around the world rely on phosphorous acid derivatives for regular spraying to keep mildew at bay and secure healthy harvests. Food producers favor compounds from this acid for low-residue, low-toxicity solutions in their integrated pest management plans. Water treatment companies mix it into corrosion inhibitors that extend pipe life and protect municipal water supplies. Some specialty manufacturers use phosphorous acid as a reducing agent for nickel-plating solutions, improving surface finish and reducing energy costs. In electronics, chemical engineers draw on it for making new phosphonate and phosphite ligands, pushing sensor and battery chemistry forward. I’ve watched companies expand research teams solely to unlock new pesticide molecules from phosphorous acid starting points, seeking both regulatory approval and a competitive edge in a crowded marketplace.
The research world stays busy with phosphorous acid and its derivatives. Universities run studies on new, less toxic compound families for crop protection. Pharmaceutical chemists test modified versions for enzyme inhibition in medical therapies; the unique properties of phosphorus-carbon bonds sometimes open doors in anti-cancer or anti-microbial research. Environmental scientists investigate low-impact formulations that minimize residual impact while keeping plant disease at bay. In green chemistry, researchers find ways to recycle phosphorus intermediates back into the supply chain, lowering environmental impact and cutting waste. The drive for more sustainable chemistry pushes labs to look at bio-based or recycled raw materials as feedstocks. Working with industry partners, I’ve seen collaborative projects where new phosphorous acid derivatives move quickly from lab bench to pilot production, offering new hope for managing global crop threats.
Scientists have looked closely at the toxicity of phosphorous acid. At industrial concentrations, direct exposure produces chemical burns and severe irritation. Its systemic products, especially when handled according to recommended guidelines, rarely leave harmful residues in food crops. Researchers continue to track the breakdown products, checking for phosphite accumulation in harvested fruits and vegetables. Agencies like the EPA and EFSA publish risk assessments, and their reviews set low thresholds for allowed residues. In controlled animal studies, high doses cause kidney and liver changes, but those doses far exceed standard agricultural exposures. Regulatory pressure keeps pushing manufacturers to reformulate products and explore lower-concentration blends that deliver the same protective effects with reduced risk. From my time studying chemical safety, I know that ongoing monitoring, worker education, and strict adherence to regulations make the difference in keeping both people and environments safe.
The future looks promising for phosphorous acid and its many derivatives. Challenges like increased crop disease outbreaks and tightening pesticide regulations steer research toward safer, more targeted molecules. Companies invest in formulation science to extend field persistence and cut application rates, tackling both environmental pressure and rising production costs. Advances in synthesis expand the range of phosphorus-containing molecules for everything from medicine to electronics. Advocates for sustainable agriculture see phosphorous acid as key to balancing plant health and food safety in a world where consumers demand both. As international standards rise and digital tracking tightens supply chain oversight, only companies with a firm grip on technical standards, safety, and science-driven innovation will thrive. From experience with industry partners, it’s clear that responding to this mix of pressure and possibility promises ongoing upgrades in both chemistry and practice.
Gardeners, orchard keepers, and even the team at the local golf course trust phosphorous acid to protect their crops and turf from some of the toughest plant diseases out there. Grapevines and potatoes often battle root rot and blight—two culprits that can kill the season’s harvest fast. Pouring money into protection that doesn’t work stings. Many now turn to foliar sprays and soil drenches made with this acid, as it targets Oomycete pathogens such as Phytophthora and Pythium. This isn’t snake oil. In studies published by university extension programs, phosphorous acid succeeded in reducing disease pressure on plants that otherwise would have withered. Farmers who depend on healthy yields every year recognize the value that dependable plant protection brings to the table, keeping food on shelves and in pantries.
So many chemicals build resistance over time. Phosphorous acid works differently. It pushes the plant’s own defenses into gear, strengthening natural immunity. Less chemical residue ends up in waterways, which matters to folks living downstream. Runoff can seriously damage aquatic ecosystems and drinking water, and farmers who worry about their neighbor’s wells trust tools that cut back on these risks. Environmental improvements like these often spring from changing how farms combat disease, not just what they use as fertilizer.
While agriculture claims much of the spotlight, some industries rely on phosphorous acid behind the scenes. Metal cleaners and stabilizers for plastics use it too. My uncle worked at a small plastics company, and he used to talk about the headaches caused by polymers breaking down too early. The acid stepped in as an antioxidant, keeping products strong and stretching their shelf life. In metal shops, the acid removes rust and preps surfaces for coatings. Quality matters to people who buy these products, and using additives that make things last longer feels like a win for everyone, not just the company’s bottom line.
Handling phosphorous acid takes respect and care. Even experienced users don’t mess around; get the concentrated stuff on your skin, and you will feel it. Spills can turn a warehouse into a hazmat call. Companies and farm crews train their staff, stock the right gear, and post instructions clearly. Most accidents come down to someone ignoring those steps. Regulatory groups stay involved, keeping tabs on storage and disposal. Safe handling keeps people, crops, and neighbors out of harm’s way, building trust in both small-town and industrial communities.
Overusing any plant treatment tends to backfire. I’ve met plenty of growers who apply phosphorous acid only as needed, not with every watering can. They keep detailed records, watch weather patterns, and adapt spray schedules if they notice runoff after heavy rain. Extension agents warn that relying on one product year after year can invite new problems. Farmers listen because their entire season rests on choices that protect both crops and land. Smarter use of phosphorous acid supports both a strong harvest and the health of land that many plan to pass along to the next generation.
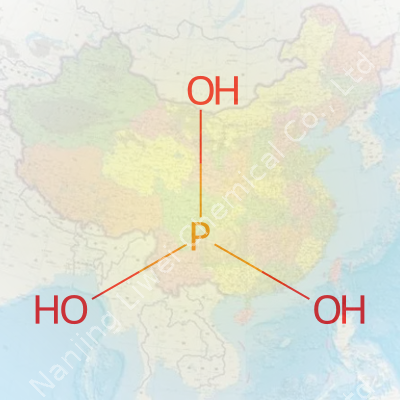
Phosphorous acid can trip up both students and professionals. Though the name sounds close to phosphoric acid, these two are not the same. The formula for phosphorous acid looks like this: H3PO3. It’s easy to jumble up the numbers or mistake it for the more widespread phosphoric acid, which uses an extra oxygen atom. This small distinction affects the acid’s properties, and those details really matter.
I remember helping with plant nutrition studies at a university greenhouse. Phosphorous acid used in some treatments arrived with clear hazard markings. Spilling it on skin or its fumes stinging the eyes left a sharp reminder about its corrosive nature. The structure of H3PO3 packs a punch partly because of the way hydrogen’s attached—two through regular bonds, one through a bond with oxygen, called a “hydroxyl group.” These hydrogen differences really change how the acid behaves in solution. It doesn’t just matter on paper; it changes real reactions and how safe you feel handling the stuff.
Phosphorous acid’s formula H3PO3 tells a story about nutrients, industry, and food security. Many specialty fertilizers include this acid because, once it breaks down, crops draw up phosphorus in the right form for root and shoot growth. Getting the formula wrong means using the wrong product in the field. Farmers have learned the hard way that results lag or crops grow poorly if formulas get mixed up. In industrial chemistry, the formula also guides engineers who monitor chemical safety and waste management.
Only a short distance separates phosphorous acid from phosphoric acid on the shelf or the order form, but their formulas differ a lot more than one letter. Phosphoric acid runs with a formula of H3PO4. Slip-ups happen, especially in busy supply chains. These errors push up costs and sometimes risk unwanted chemical reactions in storage or production. For anyone in logistics or procurement, understanding what H3PO3 means on a label can prevent lost time and unnecessary headaches.
Plenty of training sessions, whether in factories or classrooms, come back again and again to the importance of exact chemical formulas. Even one digit off in H3PO3 changes everything. Safety data sheets, emergency spill kits, and environmental protection plans tie every detail to that chemical formula. When explaining to new staff or students, I found it best to draw out the molecules and walk through each atom’s placement. Real chemistry is tactile and visual, not something to just memorize.
To avoid mistakes, labeling has to reflect the chemical formula clearly and in large print. Digital databases used in agriculture and industry should flag near matches and ask for confirmation before processing orders. Schools and university labs could lean heavier on practical demos rather than textbook drills, building knowledge from real experiments. More transparency in supply chains also matters—vendors listing the exact H3PO3 content, not just handy brand names.
No one wants wasted crops, failed reactions, or unsafe work environments. The story of phosphorous acid’s chemical formula shows why details carry so much weight in science, farming, and manufacturing. Trust in food systems, plant health, and workplace safety starts with basic chemical accuracy—down to the last number and letter.
Once, during summer lab sessions, I glanced at more than a few bottles marked “phosphorous acid.” Most folks outside of the lab might see a chemical like this and assume it’s only something for big companies or seasoned scientists. Still, garden supply catalogs and online shops now carry it, spinning stories of root health and plant disease control. Here’s where the heart of the issue lies: how much do people know about what they’re actually holding?
Phosphorous acid (H3PO3) looks clear and doesn’t smell strong—there’s no warning there. I’ve caught drops on my glove during a rushed day, and honestly, the danger hides in plain sight. It causes skin burns. Personal stories matter here: my old lab partner once ended up with raw, red marks on her wrist because some leaked under her watchband. Eyes take an even bigger hit. Even a splash, the amount you’d mistake for nothing, can scar or seriously mess with vision. Inhaling dust from dry forms makes for harsh coughing and lasting irritation.
The United States Environmental Protection Agency (EPA) lists phosphorous acid as a corrosive substance. The Centers for Disease Control and Prevention (CDC) puts it up with chemicals that require gloves, eye protection, and decent ventilation. In food production, it’s used to adjust pH but always under strict rules and with proper safeguards. The reason: nobody wants burns, breathing problems, or worse just because someone skipped basic care or wasn’t told about the risks.
I grew up in a farming town. Fertilizers and pesticides drifted on the wind. Accidental mixing or spills happened more than we’d like. Phosphorous acid doesn’t ignite easily, but it reacts with other chemicals, sometimes giving off nasty gases. Once, a neighbor mixing garden solutions in a shed coughed for a week after noticing a strange white smoke and a sour taste in the air. This isn’t rare. Improper handling can bring both immediate and slow damage—plants recover, but human lungs and skin might not.
OSHA’s material safety data points stand clear: wear nitrile gloves, goggles, and lab coats or aprons. I learned the hard way to store it away from moisture, metal, and food. Never reuse containers for anything else. Don’t dump leftover product or rinse it down a storm drain. Waterways and soil take hit after hit if this stuff seeps in regularly, making it tough for fish and plants nearby to survive.
Many backyard growers and even new employees at garden stores don’t realize what they’re working with. Information on some product labels trails behind manufacturer manuals or government advice. Some package inserts read more like fine print than a true warning. I’ve seen workshops focus more on how much to use than on what to do if something spills or splashes. Stories of kids or pets getting into the wrong bottle still turn up in local news, and the worry is real enough for families who keep chemicals in garages or basements.
Handling phosphorous acid doesn’t call for a chemistry degree—it calls for respect and careful routines. I always checked every container for leaks before use. Gloves and goggles cost less than treating a chemical burn. Training more folks, especially hobbyists and store staff, to measure solutions in well-ventilated spaces and clean up spills right away gets overlooked sometimes. Clear, bold warnings on packaging and accessible online safety resources can help. Choosing safer substitutes or professional services when possible lowers the chances of accidents. That’s not overkill—just smart practice rooted in real-world experience and years of watching what happens when shortcuts backfire.
Over the years handling different chemicals in research labs and agricultural setups, I’ve learned that phosphorous acid is not something to treat casually. This compound supports everything from plant nutrition programs to industrial applications, but its benefits come with real risks if left lying around carelessly. Simply stacking barrels in a storeroom won’t cut it—not when the stakes involve safety and environmental health.
Phosphorous acid can irritate lungs, skin, and eyes fast. I once watched a coworker get a whiff and come away coughing hard—eye protection and gloves kept the accident minor. This stuff draws moisture out of the air, so it eats through thin metal and damages cardboard packaging over time. In a hot, damp climate, I’ve seen a storeroom floor develop pools where leaky packages corroded within weeks.
Moisture transforms phosphorous acid into tricky solutions that travel fast across floors. Keeping it in air-tight containers—that means containers built for chemical storage, not just any plastic drum—has kept mishaps at bay in my experience. Any contact with metal starts slow corrosion and can alter the chemical, reducing effectiveness and triggering safety issues. Keeping containers on corrosion-resistant shelving or using non-metal bins has saved a lot of headaches.
Light and heat both speed up the breakdown of phosphorous acid, leaving white deposits or pressurized vapor. I’ve found storage in cool, shaded rooms extends shelf life and reduces weird flare-ups. I’ve opened containers left near a sunny window to find pressure build-up—always makes for a tense moment.
Nobody likes sorting through half-labeled jugs during a delivery rush, but it’s impossible to overstate how much clear labeling helps in an emergency. Every bottle on our shelves features exact contents, concentration, and hazard details. Mixing up a jug of fertilizer with one meant for cleaning could burn crops or cause a costly spill. In a shared workspace, everyone can spot the right bottle and know where to return it.
Segregating storage is another basic move. Running phosphorous acid near oxidizing materials, even for an hour, can go badly. On our shelves, we keep acids away from bases and reactives—sounds obvious, but I’ve seen newcomers store a crate of bleach too close for comfort. A physical barrier or clear shelf divider solves this problem quickly.
You can’t always control humidity or temperature, especially in rural operations, but proper ventilation flushes away any vapor or accidental releases. Simple exhaust fans and regular checks lower the risk. Nearby eyewash stations and spill kits are important; having used both, I now see them as non-negotiable, not just compliance checks.
Phosphorous acid has value for pests and accidents, so locked storage stops animals and unauthorized hands from poking around. It’s a lesson learned after finding spilled powder outside a shed one Monday morning, likely from a curious raccoon—and it could have been much worse had a child stumbled upon it.
Thoughtful, practical storage of phosphorous acid protects people and the planet. Good habits help everyone work with confidence and reduce the chance for accidents. Learning through real mistakes makes these habits second nature.
Standing in front of shelves in a lab, I can see bottles labeled phosphorous acid and phosphoric acid. On the surface, they look and sound almost identical. The differences run deeper than spelling or an extra syllable; they come down to how atoms arrange themselves and how these acids behave with other substances. Phosphoric acid holds one phosphorus atom, four oxygens, and three hydrogens (H3PO4). Phosphorous acid, on the other hand, has the formula H3PO3. Those numbers throw off more than a classroom pop quiz—they change the way farmers, chemists, and manufacturers use these acids every day.
Take phosphoric acid. It pops up in fertilizers, cleaning products, and even sodas. The food industry relies on it because it brings tartness to drinks and helps control pH. For plants, it delivers a steady punch of phosphorus, which drives growth and root development. In my work with hydroponics, liquid formulations of phosphoric acid gave better yield, showing just how much plants benefit from the extra nutrient boost.
Phosphorous acid plays in a different league. The catch: it isn’t a strong acid. It acts as a reducing agent, more suited for combating plant diseases and supporting foliar sprays. A lot of growers found out the hard way that using phosphorous acid couldn’t replace phosphoric acid as a main fertilizer. Only the latter supplies phosphorus in a form that plants pull up through their roots with ease. There’s even a warning out there—using phosphorous acid as your main phosphorus source leaves crops stunted, the soil missing what it needs, and harvests lighter than they should be.
Both acids ask for care. Phosphoric acid in cleaning agents can eat through skin or eyes on contact. Big companies put in strict protocols and personal protective equipment when using this compound to strip away hard water stains or rust. Home users aren’t always as careful, thinking “food safe” means safe across the board—but concentrated forms burn. Phosphorous acid offers similar risks, especially during mixing or dilution, so gloves and goggles never go out of style in the lab or greenhouse.
All of this connects back to regulations. Government agencies track how these acids get stored and handled, and they keep records about accidental exposure. Studies from the National Institute for Occupational Safety and Health back up those protocols. Safer handling translates directly to fewer injuries and cleaner workspaces.
Looking at farming, the stakes go beyond the lab bench. Farmers want bang for their buck, and they need to know what each acid brings. Some suppliers mix them up or use confusing names like “phosphite” or “phosphate.” I’ve seen growers misapply products, their fields underfed and sickly. The answer runs through education: clear labeling, honest supplier communication, and community workshops help close the knowledge gap. Industry groups publishing guides and demonstration plots in real farm settings help clear up years of misunderstandings.
The right acid, in the right place, changes outcomes. Misunderstandings around these two acids trip up new growers and lab techs alike. Breaking down what makes each unique—not just in a chemistry textbook, but in how they shape crops, products, and safety—becomes one of those small details that makes all the difference.
| Names | |
| Preferred IUPAC name | phosphonic acid |
| Other names |
Phosphonic acid Orthophosphorous acid |
| Pronunciation | /fɒsˈfɔːrəs ˈæsɪd/ |
| Identifiers | |
| CAS Number | 10294-56-1 |
| Beilstein Reference | 0639619 |
| ChEBI | CHEBI:28659 |
| ChEMBL | CHEMBL50415 |
| ChemSpider | 713 |
| DrugBank | DB01428 |
| ECHA InfoCard | ECHA InfoCard: 100.028.888 |
| EC Number | 231-633-2 |
| Gmelin Reference | 778 |
| KEGG | C18737 |
| MeSH | D010758 |
| PubChem CID | 24460 |
| RTECS number | TH3500000 |
| UNII | 7KJ0867QVK |
| UN number | UN2834 |
| CompTox Dashboard (EPA) | DTXSID2020715 |
| Properties | |
| Chemical formula | H3PO3 |
| Molar mass | 82.00 g/mol |
| Appearance | White crystalline solid |
| Odor | Odorless |
| Density | 1.651 g/cm³ |
| Solubility in water | Very soluble |
| log P | -1.26 |
| Vapor pressure | <0.01 mmHg (20°C) |
| Acidity (pKa) | 1.3 |
| Basicity (pKb) | 1.3 |
| Magnetic susceptibility (χ) | -63.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.384 |
| Viscosity | 1.326 mPa.s (25 °C) |
| Dipole moment | 1.13 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 110.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −1045 kJ mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1271 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | V09CX03 |
| Hazards | |
| Main hazards | Corrosive, causes severe skin burns and eye damage, harmful if swallowed, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H290, H314 |
| Precautionary statements | P260, P264, P271, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P312, P330, P363, P405, P501 |
| NFPA 704 (fire diamond) | 3-0-1-W |
| Autoignition temperature | 300°C |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 (oral, rat): 1895 mg/kg |
| LD50 (median dose) | LD50 (median dose): Acute Oral LD50 (rat): 1895 mg/kg |
| NIOSH | MN0800000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 250 mg/L |
| Related compounds | |
| Related compounds |
Hypophosphorous acid Phosphoric acid Phosphine Phosphates Phosphite |